PRESCRIBE UBRELVY® TO TREAT MIGRAINE ATTACKSWHEN HEAD PAIN SPIKES OR BEFORE IT STRIKES1
WHEN IT COMES TO TREATMENT WITH UBRELVY® FOR THE ACUTE TREATMENT OF MIGRAINE,SEE THE DATA
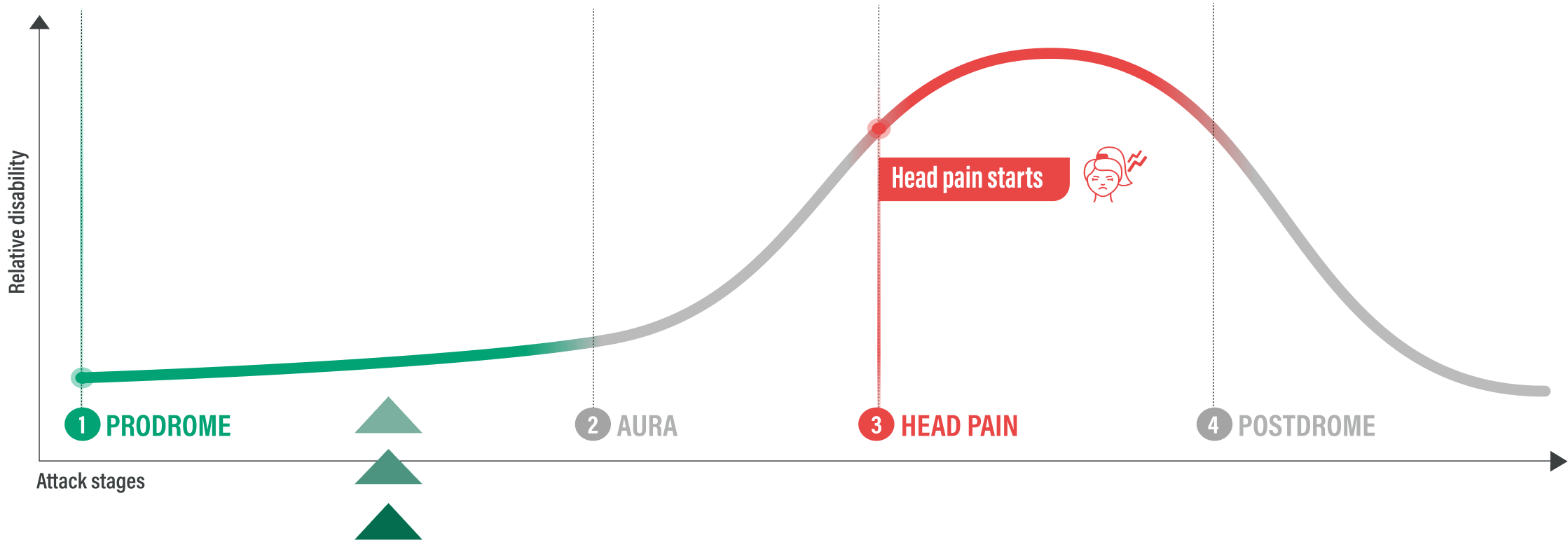
When Head Pain Spikes
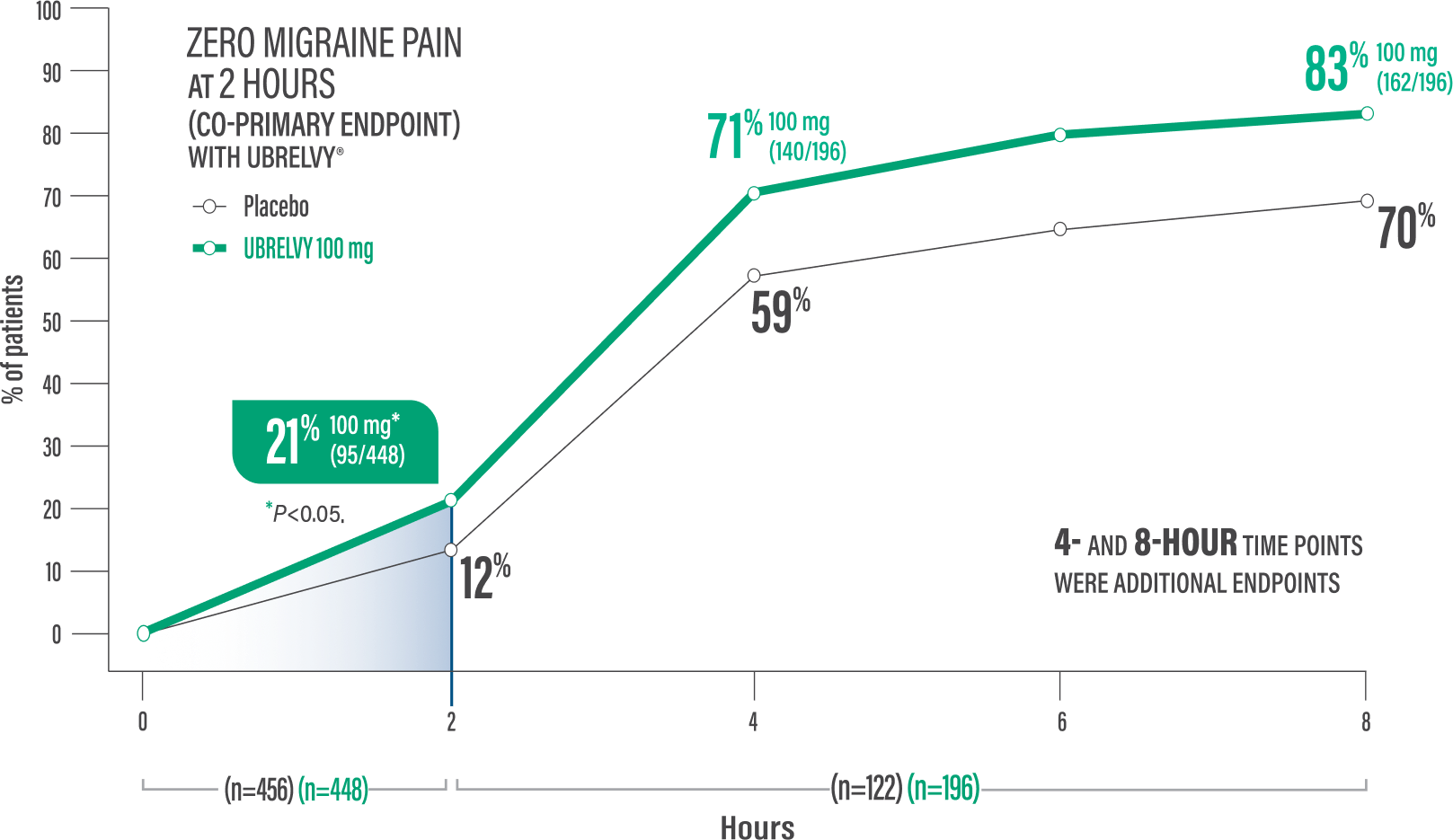
POWERFUL ELIMINATION OF MIGRAINE PAIN WITH A SINGLE DOSE WITHIN 2 HOURS
Co-primary endpoint:
Pain freedom at 2 hours with a single dose1,2*
21% of patients UBRELVY® 100 mg (95/448) vs 12% placebo (54/456)1,2†
†P<0.001.1
Additional endpoint:
Pain freedom at 4 hours with a single dose2*
71% of patients UBRELVY 100 mg (140/196) vs 59% placebo (72/122)2
LIMITATIONS: The analyses of additional endpoints were not tested in hierarchical order or adjusted for multiplicity. Therefore, results cannot be regarded as statistically significant. The analyses of additional endpoints were censored to exclude data collected after the second dose and rescue medication.
Data presented above are in reference to UBRELVY 100 mg only. See similar results for patients taking UBRELVY 50 mg.2 See ACHIEVE pivotal trials design pop-up for more detail, including additional co-primary endpoint data.
*Pain freedom was defined as a reduction from moderate or severe headache pain to no pain.1
When Head Pain Spikes
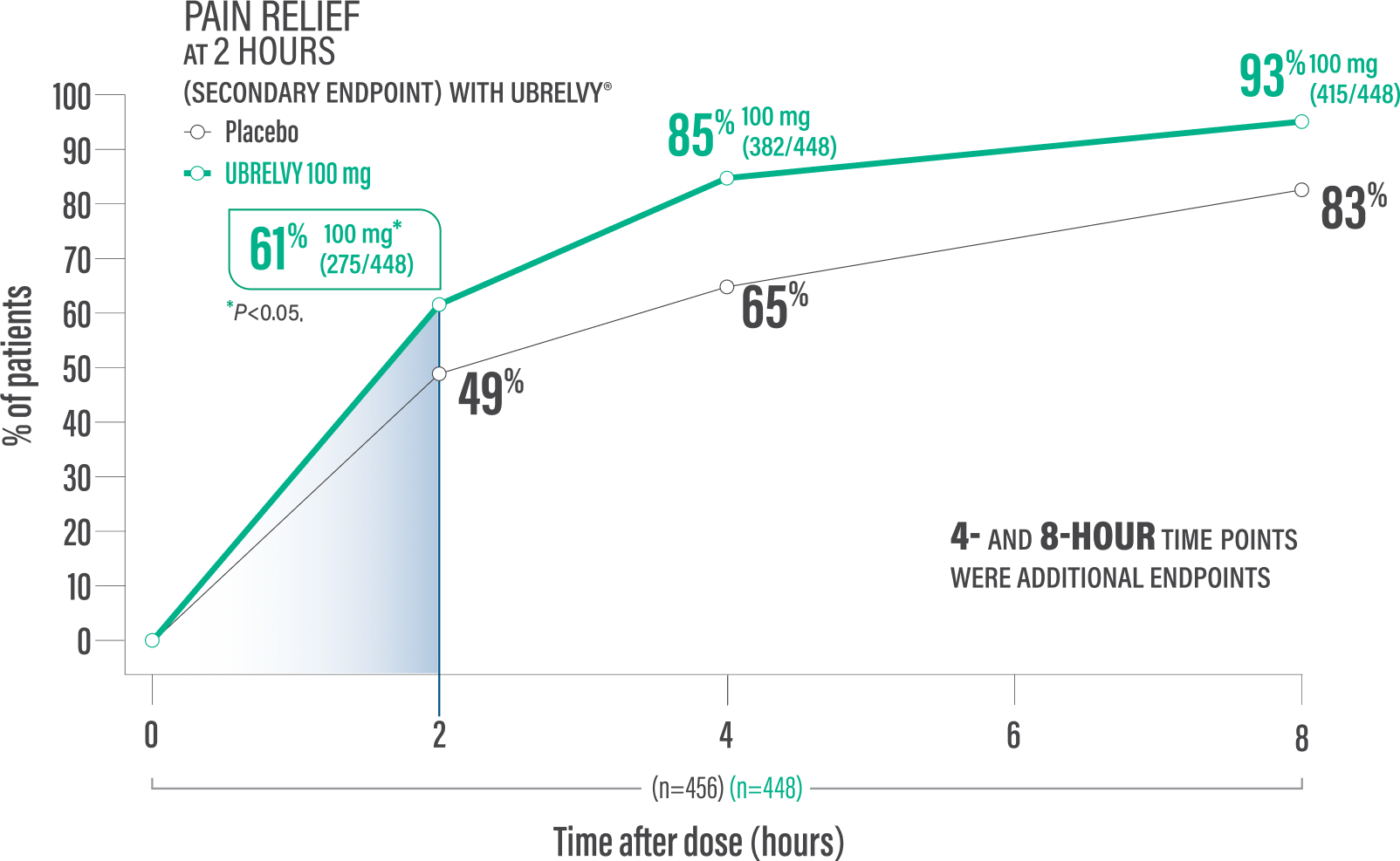
UBRELVY DELIVERED RAPID PAIN RELIEF WITHIN 2 HOURS1
Secondary endpoint:
Pain relief at 2 hours with a single dose4*
61% of patients 100 mg (275/448) vs 49% placebo (224/456)1†
†P<0.05.1
Additional endpoint:
Pain relief at 4 hours with a single dose2*
85% of patients 100 mg (382/448) vs 65% placebo (298/456)2
LIMITATIONS: The analyses of additional endpoints were not tested in hierarchical order or adjusted for multiplicity. Therefore, results cannot be regarded as statistically significant. The analyses of additional endpoints were censored to exclude data collected after the second dose and rescue medication.
Data presented above are in reference to UBRELVY 100 mg only. See the 50 mg results pop-up for similar results for patients taking UBRELVY 50 mg.2,5 See ACHIEVE pivotal trials design pop-up for more detail, including additional co-primary endpoint data.
*Pain relief was defined as a reduction in migraine pain from moderate or severe to mild or none postdose.1
When Head Pain Spikes
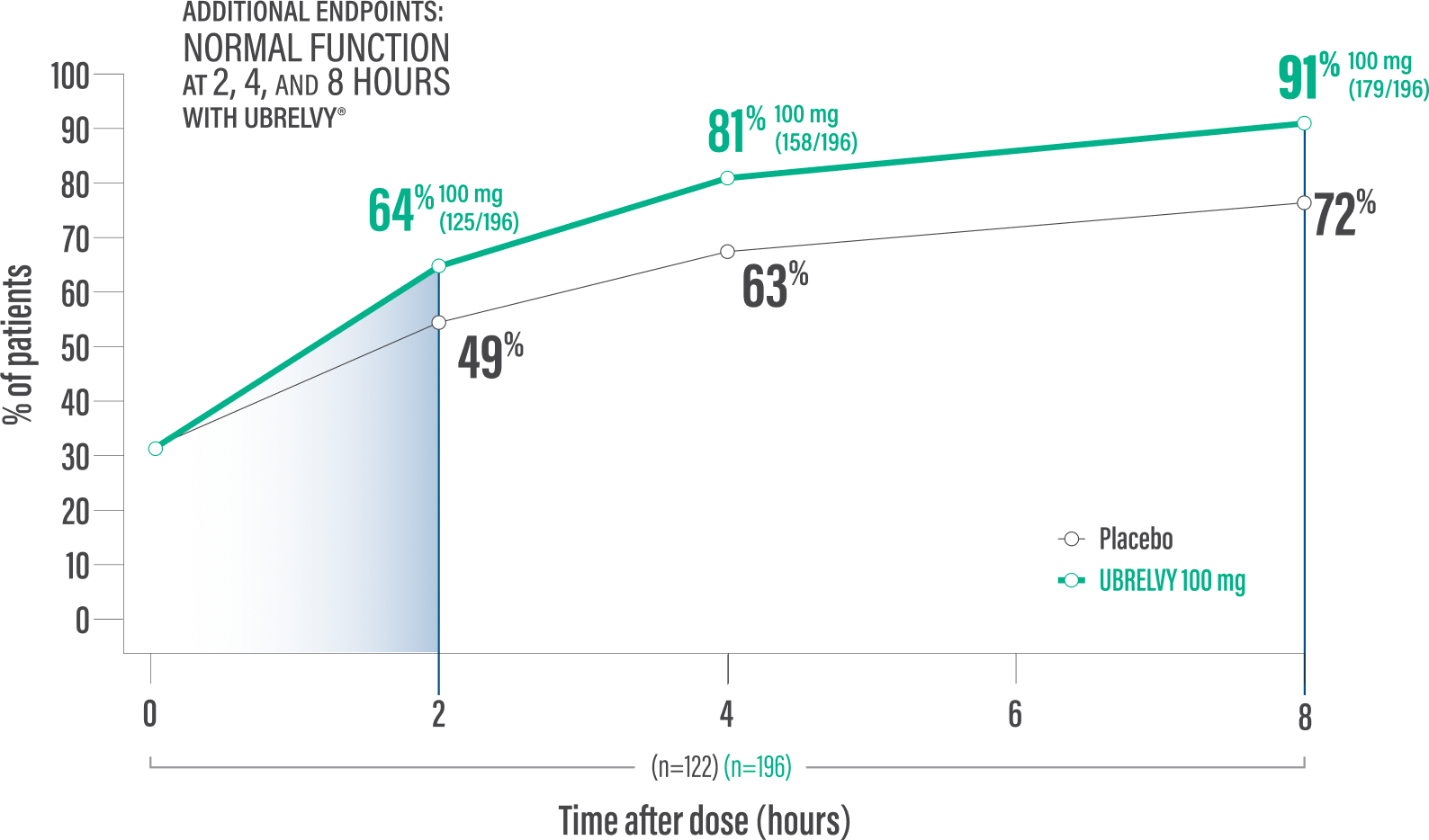
RETURN TO NORMAL FUNCTION DATA WITH UBRELVY2
Additional endpoint:
Normal function* at 2 hours with a single dose6
64% of patients 100 mg (125/196) vs 49% placebo (60/122)2
Additional endpoint:
Normal function* at 4 hours with a single dose6
81% of patients100 mg (158/196) vs 63% placebo (77/122)2
Data presented above are in reference to UBRELVY 100 mg only. See the 50 mg results pop-up for similar results for patients taking UBRELVY 50 mg.2 See ACHIEVE pivotal trials design pop-up for more detail, including additional co-primary endpoint data.
LIMITATIONS: The analyses of additional endpoints were not tested in hierarchical order or adjusted for multiplicity. Therefore, results cannot be regarded as statistically significant. The analyses of additional endpoints were censored to exclude data collected after the second dose and rescue medication.
*Patients were asked to rate the performance of daily activities using 4 response options ranging from 0 (no disability, able to function normally) to 3 (severely impaired, cannot do all or most things, bed rest may be necessary).6
When Head Pain Spikes
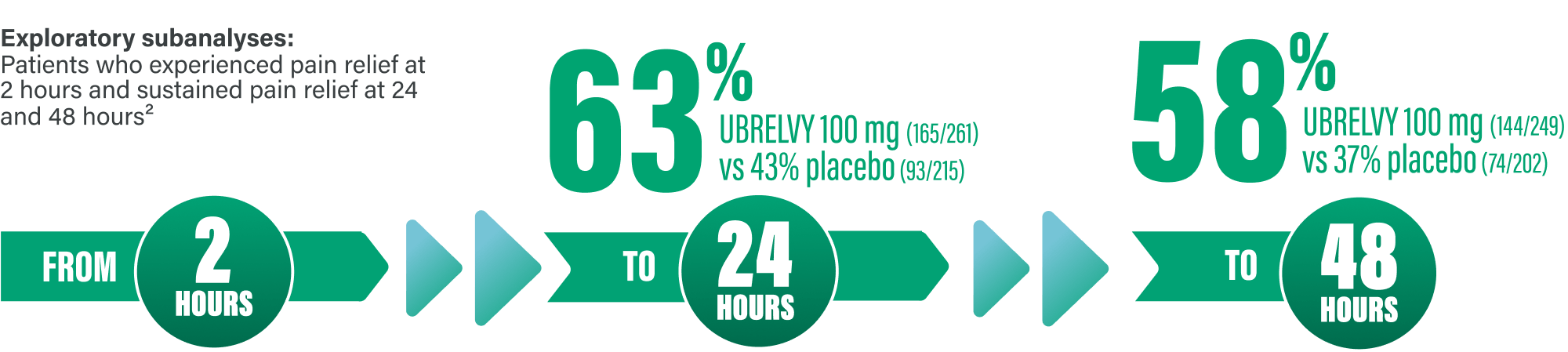
SUSTAINED PAIN RELIEF DATA WITH UBRELVY FROM AN EXPLORATORY SUBANALYSIS1
61% of patients achieved pain relief at 2 hours (100 mg: 275/448) vs 49% with placebo (224/456)1
IN THE SUBSET OF PATIENTS WHO EXPERIENCED PAIN RELIEF AT HOUR 2, PAIN RELIEF WAS SUSTAINED IN A MAJORITY OF PATIENTS1*
LIMITATIONS: Data for these post hoc analyses are descriptive only and no statistical conclusions can be made from these results. As these data only include patients experiencing pain relief at 2 hours, subsequently evaluated at 24 and 48 hours, these data are enriched and should be interpreted with caution. Participants with missing data after 2 hours were not included in the exploratory subanalysis. Results should be interpreted with these factors in mind.
*Sustained pain relief from 2 to 24 hours was defined as pain relief with no administration of either rescue medication or second dose of investigational product, and no occurrence thereafter of moderate or severe headache pain during the relevant number of hours after dosing.7
Data presented above are in reference to UBRELVY 100 mg only. See the 50 mg results pop-up for similar results for patients taking UBRELVY 50 mg.2,5 See ACHIEVE pivotal trials design pop-up for more detail, including co-primary endpoint data.
Before Head Pain Strikes
THOSE WHO TOOK UBRELVY AT THE EARLY SIGNS OF A MIGRAINE ATTACK
AVOIDED INTENSE HEAD PAIN BEFORE IT STARTED 3
Primary endpoint:
Absence of headache pain of moderate to severe intensity within 24 hours after taking UBRELVY 100 mg in the prodrome phase (defined in the study as 1-6 hours prior to onset of headache)3
| 46%‡ | OF PATIENTS WHO TOOK UBRELVY 100 mg IN THE PRODROME PHASE (190/418) VS 29% PLACEBO (121/423) AVOIDED INTENSE HEAD PAIN BEFORE IT STARTED THROUGH 24 HOURS3 ‡P<0.0001 vs placebo.3 |
Safety in the prodrome trial was consistent with pivotal clinical trials1,3
The most common TEAEs (≥2% in either group) were nausea (UBRELVY 100 mg: 5% vs placebo: 3%), dizziness (2% vs 3%), fatigue (3% vs 2%), and somnolence (2% vs 1%).#
LIMITATIONS: This study evaluated a subset of the migraine population who could reliably predict the onset of migraine headache pain (per clinician judgment) within a short time window. Patients should be carefully assessed on their ability to accurately predict the onset of a migraine attack and progression to the headache phase. The 50 mg dose was not assessed. Patients were not allowed to administer a second dose.
Study Design3: A phase 3, multicenter, randomized, double-blind, placebo-controlled, crossover study that evaluated the absence of headache pain of moderate to severe intensity within 24 hours postdose after taking a single dose of UBRELVY 100 mg during the prodrome phase. See prodrome study design pop-up for additional details.
mITT=modified intent-to-treat; TEAE=treatment-emergent adverse event.
§No conclusions can be made about the efficacy of UBRELVY for prodrome symptoms.
||Odds ratio (95% CI) was based on a generalized linear mixed model with treatment group and treatment period as categorical fixed effects.3
¶The mITT population consisted of all randomized participants with at least 1 assessment of headache occurrence within 24 hours after taking double-blind study intervention for at least 1 qualifying prodrome event during the double-blind treatment period.3
#TEAEs reflected any adverse events reported within 48 hours after taking UBRELVY or placebo.3