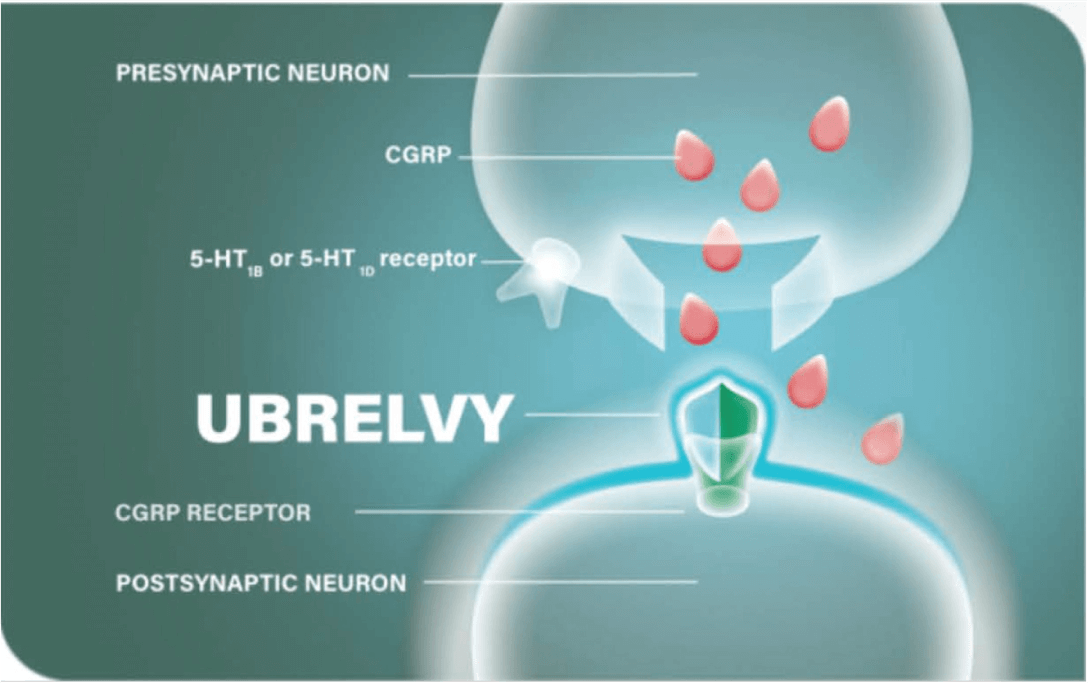
UBRELVY® is the first and only acute treatment for migraine that has demonstrated data in the prodrome phase in a phase 3, double-blind, placebo-controlled trial.1,2
Key inclusion criteria1,2
This study was conducted for patients with migraine who recognized prodrome symptoms of a migraine attack 1 to 6 hours before the headache phase. Patients were between 18 and 75 years of age with ≥1-year history of migraine (with or without aura), consistent with a diagnosis according to the ICHD-3. Migraine onset occurred before 50 years of age. Migraine attacks occurred 2 to 8 times/month with moderate to severe headache, typically separated by 48 hours and lasting between 4 and 72 hours if untreated or treated unsuccessfully. Patients had current or past use of ≥1 prescription medication for the acute treatment of migraine or preventive treatment. Patients were eligible for randomization in the double-blind treatment period if ≥75% of 4 to 16 migraine attacks with prodrome symptoms led to headache pain (of any intensity) within 1 to 6 hours or, if only 3 migraine attacks with prodrome symptoms were identified, all were required to lead to headache 100% of the time.
HCP interview questions to determine patient eligibility:
- “Do you have ‘warning signs’ that tell you when a headache is about to start? Describe them for me.”
- “Considering all your migraine headaches, what percentage are preceded by prodrome/warning symptoms?”
- “After experiencing your prodrome symptoms, how reliably does a headache occur within 1 to 6 hours?”*
77% of patients (n=911) reliably identified their prodrome symptoms, which included, but were not limited to, sensitivity to light (57%), fatigue (50%), neck pain (42%), sensitivity to sound (34%), and dizziness (28%).1*†
After meeting eligibility criteria, randomized patients (n=518) treated 2 separate migraine attacks with prodrome symptoms (≥7 days apart), one with UBRELVY 100 mg and one with placebo.1‡
Participant flow2
1087 patients were screened for eligibility and 479 patients discontinued due to screening failure, including failure to meet inclusion criteria (317) and failure to screen due to a qualifying event (290).
References: 1. Dodick DW, Goadsby PJ, Schwedt TJ, et al. Ubrogepant for the treatment of migraine attacks during the prodrome: a phase 3, multicentre, randomised, double-blind, placebo-controlled, crossover trial in the USA. Lancet. 2023;402(10419):2307-2316. 2. Data on file. AbbVie Inc.