Zoe
"When a migraine strikes and I've taken a triptan, side effects like dizziness8-14 can throw me off."
UBRELVY works differently. It directly blocks CGRP, which is linked to causing migraine.2,15
In pivotal clinical studies, patients took UBRELVY within 4 hours of migraine headache onset.1 21% of patients experienced pain freedom at 2 hours (co-primary endpoint). UBRELVY 100 mg (95/448) vs 12% placebo (54/456).2,3 Pain freedom was defined as a reduction from moderate or severe headache pain to no pain.1 See similar results for patients taking UBRELVY 50 mg.4 See ACHIEVE pivotal trials design for more detail, including additional co-primary endpoint data.
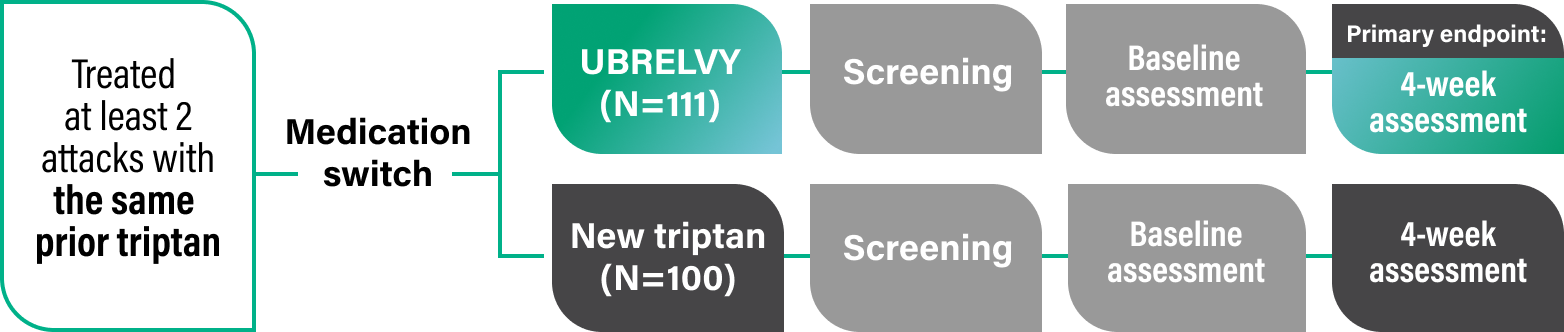
The UNIVERSE II survey study was a prospective, observational, longitudinal real-world study comparing patient-reported outcomes in the acute treatment of migraine in the United States.5
Objective: Evaluate patient-reported outcomes in participants with migraine who switched from a prior oral triptan* either to another oral triptan or to UBRELVY. Patients were followed for a period of 4 weeks and outcomes were collected via the Migraine Buddy app.5,6
Migraine Buddy app: Migraine Buddy is a free smartphone app that is widely used by individuals worldwide to self-report their patterns of migraine, characteristics, and coping mechanisms by regularly logging their migraine details into the app, including triggers, symptoms, and impact of migraine on their daily lives.6
Eligible participants who met the screening criteria were invited to participate in the study by completing an electronic Informed Consent Form (ICF). After completing the ICF, participants were directed to complete a baseline assessment the next day in an electronic diary format in the Migraine Buddy app. Participants then received daily reminders to complete daily assessments for the next 4 weeks (days 2-29). The final assessment was completed on day 30.6
The Migraine Buddy application undergoes regular quality control testing to ensure it runs as intended on all platforms. User Acceptance Testing (UAT) is performed to ensure that the questionnaires display as intended, the survey flow works as specified in the protocol, and that the participant responses properly feed the study database. No “ease of use” testing was performed in the target population. Evidence of content validity (eg, relevance of the items, comprehensibility, clarity) of the satisfaction and treatment preference survey questions has not been established. Participants using the Migraine Buddy app might have more severe or frequent migraine attacks than patients who do not feel the need to use an app for recording personal health information about their migraine attacks. The results of this study may not be generalizable to the general population.6
Change from baseline at 4 weeks included6:
Eligibility criteria5:
Patient-reported outcome assessments at 4 weeks included5:
Preventive medication use was assessed at baseline and at week 46
There were also optional Daily Diary Assessments on days 2-296
Additional considerations:
Survey questions to determine satisfaction with treating pain associated with migraine and treatment preference6:
WPAl=Work Productivity and Activity Impairment questionnaire.
*Triptans are a class of prescription acute treatments for migraine.5
†Sensitivity to light (triptan, 34.0%; UBRELVY, 35.1%), and pain with routine activities (triptan, 19.0%; UBRELVY, 14.4%; excluding head pain) were reported as the most bothersome migraine symptoms in both cohorts.5
‡Responders were defined as patients who reported they were 'extremely satisfied' or 'satisfied' on a 7-point Likert scale.6
§Responders were defined as patients who reported they were 'a little more satisfied' or 'a lot more satisfied' with their new medication compared to their previous triptan.5
Limitations:
No head-to-head trials have been conducted between UBRELVY and triptans, therefore no conclusions regarding safety or efficacy can be drawn. Data collected may be subject to bias and recall error.6
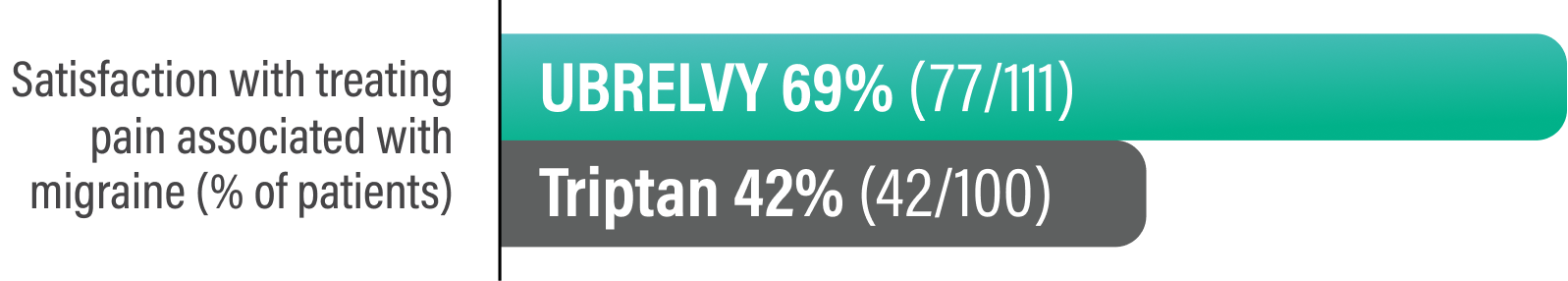
Survey Question:
Overall, how satisfied are you with your UBRELVY/Triptan for treating pain associated with your migraine? (on a 7-point Likert scale ranging from 'extremely satisfied' to 'extremely dissatisfied.' Responders included those who selected 'extremely satisfied' or 'satisfied.')6
LIMITATIONS5,6: The results represent real-world, observational treatment utilization and outcomes and do not describe results from randomized controlled/head-to-head clinical trials. No causal relationships, including conclusions related to comparative clinical efficacy and safety, can be drawn from study results. There was no planned statistical procedure to adjust for multiplicity, so there was no control of the overall Type 1 error rate (false positive rate). The analyses do not ascertain whether these findings were attributable to treatment with UBRELVY, rather than merely due to chance, and are susceptible to bias. All observations were self-reported, and study assessments were designed to gather responses that occurred within the last 1 week to 4 weeks, which introduced the possibility of recall error.
No head-to-head trials have been conducted between UBRELVY and triptans, therefore no conclusions regarding safety or efficacy can be drawn. Data collected may be subject to bias and recall error.2
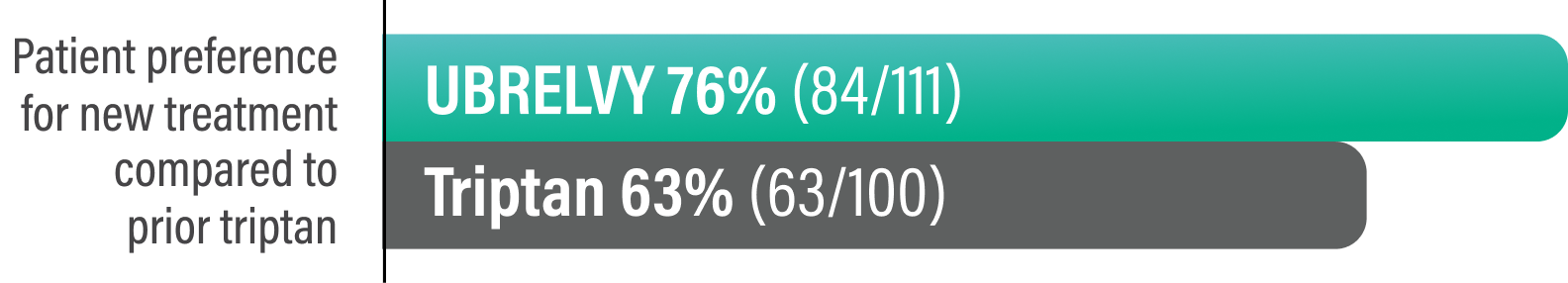
Survey Question:
Compared to your previous treatment, which medication do you prefer? (on a 5-point scale ranging from 'prefer previous triptan - I was a lot more satisfied' to 'prefer [new triptan/UBRELVY] - I am a lot more satisfied.’ Responders included those who selected 'prefer [new triptan/UBRELVY] - I am a lot more satisfied or I am a little more satisfied.')6
LIMITATIONS5,6: The results represent real-world, observational treatment utilization and outcomes and do not describe results from randomized controlled/head-to-head clinical trials. No causal relationships, including conclusions related to comparative clinical efficacy and safety, can be drawn from study results. There was no planned statistical procedure to adjust for multiplicity, so there was no control of the overall Type 1 error rate (false positive rate). The analyses do not ascertain whether these findings were attributable to treatment with UBRELVY, rather than merely due to chance, and are susceptible to bias. All observations were self-reported, and study assessments were designed to gather responses that occurred within the last 1 week to 4 weeks, which introduced the possibility of recall error.
THE TRIPTAN-EXPERIENCED PATIENT: ZOE IS UBRELVY® -READY

Zoe
"When a migraine strikes and I've taken a triptan, side effects like dizziness8-14 can throw me off."
UBRELVY works differently. It directly blocks CGRP, which is linked to causing migraine.2,15
Not an actual patient.
Model and quote are for illustrative purposes only.
CGRP=calcitonin gene-related peptide.
WHO IS ZOE?
TREATMENT JOURNEY
TREATMENT GOALS

IS IT TIME TO START UBRELVY INSTEAD OF ANOTHER TRIPTAN?
These are patient perceptions around the experience of taking UBRELVY
WISHED THEY HAD STARTED UBRELVY SOONER
IN THEIR MIGRAINE TREATMENT JOURNEY 16
FOUND THAT UBRELVY WAS EASY TO GET FROM
THE PHARMACY AND TO AFFORD ONCE PRESCRIBED 17
BELIEVED THEY COULD GO ON WITH
THE REST OF THEIR DAY WHEN TAKING UBRELVY 18
||Source: Online survey conducted by Ipsos Healthcare on behalf of AbbVie Inc., between August 20, 2024, and September 24, 2024, among 500 adults aged 18-64, of which 200 were using UBRELVY at the time of the survey.16-18
¶Survey respondents could choose from the following to indicate their agreement with the provided statement: strongly disagree, disagree, somewhat disagree, neither disagree nor agree, somewhat agree, agree, or strongly agree. The percentage represents those who answered somewhat agree, agree, and strongly agree.16-18
CONTRAINDICATIONS
UBRELVY is contraindicated:
Hypersensitivity Reactions: Cases, including anaphylaxis, dyspnea, facial or throat edema, rash, urticaria, and pruritus, have been reported. Hypersensitivity reactions can occur minutes, hours, or days after administration. Most reactions were not serious, and some led to discontinuation. If a serious or severe reaction occurs, discontinue UBRELVY and institute appropriate therapy.
Hypertension (HTN): Development or worsening of pre-existing HTN has been reported following the use of CGRP antagonists, including UBRELVY. Some patients who developed new-onset HTN had risk factors. There were cases requiring initiation of HTN treatment and, in some cases, hospitalization. HTN may occur at any time but was most frequently reported within 7 days of initiation. The CGRP antagonist was discontinued in many of the cases. Monitor patients for new-onset or worsening of pre-existing HTN and consider whether discontinuation of UBRELVY is warranted if evaluation fails to establish an alternative etiology or blood pressure is inadequately controlled.
Raynaud’s phenomenon (RP): Development, recurrence, or worsening of pre-existing RP has been reported following the use of CGRP antagonists, including UBRELVY. In cases with small molecule CGRP antagonists, symptom onset occurred a median of 1.5 days following dosing. Many of the cases reported serious outcomes, including hospitalizations and disability, generally related to debilitating pain. In most cases, discontinuation of the CGRP antagonist resulted in resolution of symptoms. UBRELVY should be discontinued if signs or symptoms of RP develop, and patients should be evaluated by a healthcare provider if symptoms do not resolve. Patients with a history of RP should be monitored for, and informed about the possibility of, worsening or recurrence of signs and symptoms.
ADVERSE REACTIONS
The most common adverse reactions were nausea (4% vs 2% placebo) and somnolence (3% vs 1% placebo).
DRUG INTERACTIONS
UBRELVY® (ubrogepant) is indicated for the acute treatment of migraine with or without aura in adults. UBRELVY is not indicated for the preventive treatment of migraine.
US-UBR-250101
References: 1. Dodick DW, Lipton RB, Ailani J, et al. Ubrogepant, an acute treatment for migraine, improved patient-reported functional disability and satisfaction in 2 single-attack phase 3 randomized trials, ACHIEVE I and II. Headache. 2020;60(4):686-700. 2. UBRELVY [package insert]. North Chicago, IL: AbbVie Inc.; 2025. 3. Data on file. ACHIEVE I and ACHIEVE II Pain Freedom Timecourse Tables. AbbVie Inc. 4. Hutchinson S, Dodick DW, Treppendahl C, et al. Ubrogepant for the acute treatment of migraine: pooled efficacy, safety, and tolerability from the ACHIEVE I and ACHIEVE II phase 3 randomized trials. Neurol Ther. 2021;10(1):235-249. 5. Lipton RB, Parikh K, Gao X, et al. Patient-reported outcomes in acute migraine management: a prospective, real-world, longitudinal study (UNIVERSE II) comparing migraine patients who switch from a prior triptan to another triptan or ubrogepant. Poster presented at: 66th Annual Scientific Meeting of the American Headache Society; June 13-16, 2024; San Diego, CA. 6. Data on file. Health Economics and Outcomes Research (HEOR) Study Report: Patient relevant outcomes in migraine patients who recently switched to ubrogepant or a new oral triptan medication from a prior triptan. AbbVie Inc. 7. Data on file. HEOR Study Protocol. AbbVie Inc. 8. AMERGE [package insert]. Durham, NC: GlaxoSmithKline; 2020. 9. AXERT [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2017. 10. FROVA [package insert]. Malvern, PA: Endo Pharmaceuticals; 2018. 11. IMITREX [package insert]. Durham, NC: GlaxoSmithKline; 2024. 12. MAXALT [package insert]. Jersey City, NJ: Organon LLC; 2021. 13. RELPAX [package insert]. Morgantown, WV: Viatris; 2024. 14. ZOMIG [package insert]. Bridgewater, NJ: AstraZeneca; 2019. 15. Edvinsson L, Haanes KA, Warfvinge K, Krause ON. CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat Rev Neurol. 2018;14(6):338-350. 16. Data on file. AbbVie Inc. 17. Data on file. AbbVie Inc. 18. Data on file. AbbVie Inc.